Research Funding
U.S. National Institutes of Health, Pharmaceutical/Biotech Company
Background
Tak is an oral selective nonsteroidal 17, 20-lyase inhibitor that blocks the synthesis of gonadal and adrenal androgens. We evaluated the clinical benefit of Tak with ADT in pts with newly diagnosed mHSPC.
Methods
Pts with mHSPC with a Zubrod performance status (PS) of 0-2 and a PSA of ≥ 2 ng/ml were randomized 1:1 to ADT+Tak (300 mg twice daily) or ADT+Bic (50 mg daily). Stratification factors included PS (0-1 vs ≥2), extent of disease (minimal vs extensive), and receipt of ADT prior to registration (yes vs no). The primary endpoint was overall survival (OS). Secondary endpoints were progression free survival (PFS; based on PSA, imaging or clinical progression), PSA at 7 months (≤0.2 vs 0.2 4 ng/ml) and adverse event (AE) profile. With 2.75 yrs to accrue 1,186 eligible pts and 3 additional yrs of follow-up, we would have 90% power to determine a 33% improvement in OS from 54 to 72 mos (1-sided α = 0.025). A final analysis was pre-specified after 523 deaths using a 1-sided α = 0.022 to account for interim analyses.
Results
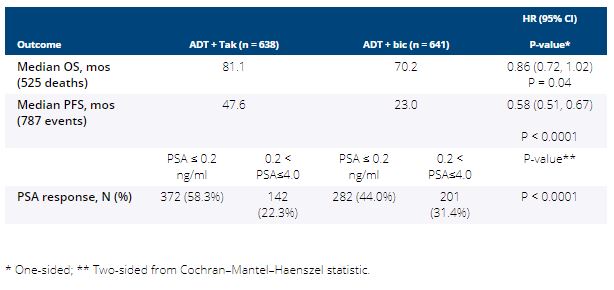
Between 3/2013 and 7/2017, 1,313 pts were randomized and 1,279 were included in the intention-to-treat (ITT) analysis (32 pts were ineligible and 2 pts withdrew consent). Median age was 68 yrs and 10% of subjects were Black. Median PSA was 30 ng/mL (range 2-6710) and 49% of pts had extensive disease. After a median follow-up of 4.9 yrs, PFS and PSA response were significantly improved with Tak over Bic but no significant improvement in OS was observed (Table). More grade 3/4 AEs occurred in Tak vs. Bic arms (43% vs. 14%), and included hypertension (20% vs. 5%) and fatigue (5% vs. 2%). Five pts in Tak and 1 pt in the Bic arm had grade 5 AE.
Conclusions
Despite clinically meaningful improvement in various outcome measures with Tak+ADT over Bic+ADT in this representative population of mHSPC, the improvement in OS did not meet the pre-specified criteria for statistical significance. The median OS of 70 mos in the control arm (standard ADT) was higher than that reported in contemporary phase 3 trials in this setting, and 16 mos higher than originally estimated. This trial sets a new landmark for survival estimates when pts with mHSPC have access to multiple approved subsequent life-prolonging therapies. Funding: NIH/NCI/NCTN grants U10CA180888, U10CA180819, U10CA180820; U10CA180821; and in part by Millennium Pharmaceuticals, Inc. (Takeda Pharmaceutical Company LTD) Clinical trial information: NCT01809691