Background
Immune therapies have offered limited efficacy in mCRPC. AMG 160 is a targeted HLE BiTE® immune therapy that engages patients’ own T cells to kill prostate cancer cells via binding of CD3 on T cells and PSMA on cancer cells. We report preliminary results from the dose exploration portion of an ongoing phase I study of AMG 160 in mCRPC.
Methods
Eligible patients (pts) had mCRPC refractory to prior novel hormonal therapy and 1–2 taxane regimens and evidence of progressive disease. AMG 160 was administered as a short IV infusion Q2W at doses of 0.003–0.9 mg. The combination of AMG 160 + pembrolizumab was also evaluated. Study objectives were to evaluate safety, tolerability, pharmacokinetics, and anti-tumor activity.
Results
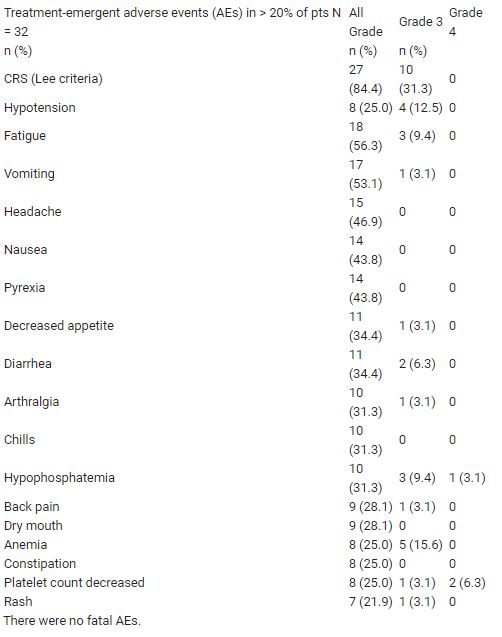
As of 11 May 2020, 32 pts had received ≥ 1 dose of AMG 160 monotherapy at 6 dose levels (DL), and 16 pts were on treatment (6 for ≥ 6 mo). Median (range) age was 65.5 (49–78) y; 20 pts (63%) had received > 3 prior lines of therapy. Cytokine release syndrome (CRS), the most common adverse event (27 pts, see Table), presented with fever, transaminitis, and hypotension, occurred primarily in cycles 1–2, and was managed with standard mitigation approaches. One treatment-related discontinuation (DL5) and 2 reversible dose-limiting toxicities (DLTs) were reported: rash (DL3) and GI hemorrhage (DL6). MTD has not been reached. RECIST 1.1 responses among the 18 pts with measurable disease included 1 confirmed PR (DL4), 5 SD, and 5 PD. PSA reductions occurred in 15/24 (63%) evaluable pts; reductions >50% occurred in 6/10 (60%) pts at DL5 and DL6. Overall, 6/22 (27%) pts had confirmed PSA responses: 1 PSA90 (DL4), 3 PSA50 (DL3, DL5, DL6), 2 PSA30 (DL3, DL5). Six pts also received AMG 160 (DL3) + pembrolizumab; no DLTs were reported.
Conclusions
AMG 160 treatment was tolerable with preliminary efficacy. Further dose exploration is ongoing; results will be updated.
Table: 609O