Research Funding
AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth,NJ, USA
Background
Not all mCRPC patients have available or sufficient tissue for multigene molecular testing. In the Phase 3 PROfound study, olaparib significantly improved radiographic progression-free survival compared with physician’s choice of abiraterone or enzalutamide in men with homologous recombination repair (HRR)-gene-mutated mCRPC (de Bono et al. N Engl J Med 2020). Overall, 31% of patients’ tissue samples failed molecular screening during the study, showing the need for additional testing methods to detect patients with HRR-gene-mutated cancers. We evaluated the utility of plasma-derived ctDNA to identify deleterious BRCA and ATM mutations in screened patients from PROfound.
Methods
Tumour samples were prospectively tested at Foundation Medicine, Inc (FMI) using an investigational next-generation sequencing test (based on FoundationOne CDx) to inform trial eligibility. Matched ctDNA samples were sequenced at FMI with the FoundationOne Liquid CDx assay. Tissue samples were clinically heterogeneous regarding location and timing of collection; plasma samples were collected as part of screening in PROfound.
Results
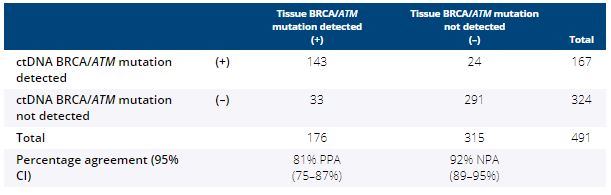
81% (503/619) of ctDNA samples tested yielded a result, of which 491 had a tumour result. BRCA and ATM status in tissue compared with ctDNA reported 81% (95% CI 75–87%) positive percentage agreement (PPA) and 92% (95% CI 89–95%) negative percentage agreement (NPA), with tissue as reference (Table). Further concordance and discordance measures will be presented.
Conclusions
High concordance between tumour tissue and ctDNA supports the development of ctDNA testing as a minimally invasive method to identify patients with HRR-gene-mutated mCRPC and guide treatment decisions, particularly for those with insufficient tissue for genomic analyses. Clinical trial information: NCT02987543.