Background
NEPC is a high-grade aggressive form of prostate cancer. We queried whether RB1 mutation status would impact the genomic features of NEPC. We hypothesized that there would be differences in GA frequencies in RB1 mutated vs non mutated cases.
Methods
From a series of 13,496 cases of clinically advanced PC, 415 (3.1%) histologically defined NEPC were sequenced using a hybrid capture-based FDA-approved clinical genomic profiling (CGP) assay to detect all classes of genomic alterations (GA). Tumor mutational burden (TMB) was determined on 0.8 Mbp of sequenced DNA and microsatellite instability (MSI) was determined on 95 loci. PD-L1 expression was determined by IHC (Dako 22C3) with low tumor cell positive staining 1-49% and high staining ≥50% expression.
Results
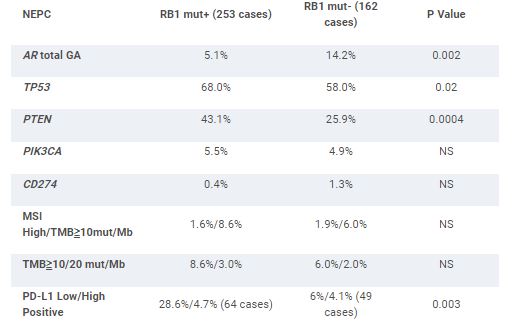
253 (61%) of NEPC feature GA in RB1 (RB1 mut+). This contrasts with a 5.8% frequency of RB1 GA in the non-NEPC (P < .0001). The RB1 mut+ NEPC featured a slightly greater number of driver GA/tumor than the RB1 mut- NEPC (5.1 vs 4.2). Age and TMPRSS2-ERG fusion frequency were similar between the groups. RB1 Mut- NEPC was associated with significantly higher amplifications (amp) and total GA in AR compared to RB1 mut+ NEPC. RB1 mut+ NEPC featured significantly greater frequency of PTEN GA. GA frequencies in targetable kinases and DNA repair GA including BRCA1/2 and ATM linked to PARP inhibitor (PARPi) response were similar in both groups. For potential immune-oncology (IO) biomarkers, RB1 Mut+ NEPC featured significantly greater frequency of low positive PD-L1 expression and lower frequencies of MDM2 and CDK12 GA. CD274 (PD-L1) amplification, MSI-high status and cases with TMB ≥10 mut/Mb were very uncommon in both groups.
Conclusions
In NEPC, the presence of RB1 mutation is associated with various GA that may have clinical relevance. Limitations include possible selection and confounding biases, and lack of clinical annotation. Further study of RB1 status as a guide of trial designs on therapy selection for men with NEPC is warranted.