Research Funding
Janssen Research & Development
Background
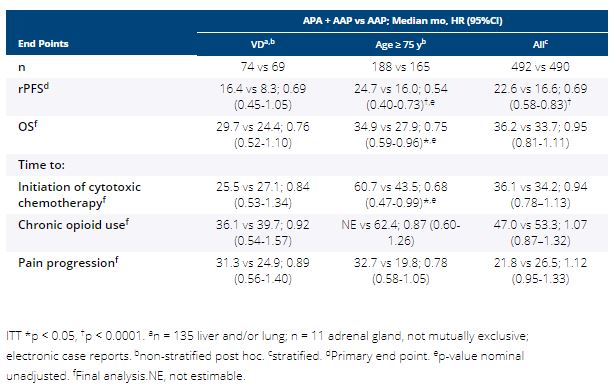
In the double-blind PBO-controlled ACIS study, investigator-assessed radiographic progression-free survival (rPFS) was significantly improved with APA + AAP vs PBO + AAP in chemo-naive mCRPC, with no significant new safety signals (Rathkopf ASCO GU 2021). Among prespecified subgroups, efficacy and safety were explored in two difficult to treat subgroups: pts with visceral disease (VD; liver, lung, and/or adrenal gland metastasis) or age ≥ 75 y.
Methods
Pts with mCRPC with ongoing ADT and no prior life-prolonging treatment were randomized 1:1 to APA (240 mg QD) + AA (1000 mg QD) + P (5 mg BID) or PBO + AAP. Stratified: presence or absence of VD, ECOG PS 0 or 1, geographic region. Primary end point: rPFS (randomization to radiographic progression or death); secondary end points: overall survival (OS), time to initiation of cytotoxic chemotherapy, time to chronic opioid use, time to pain progression, safety.
Results
982 pts enrolled. 14.6% had VD and 35.9% were ≥ 75 y. Median rPFS, OS, and time to pain progression favored APA + AAP vs AAP (HR 94%) in pts with VD, ≥ 75 y, and overall safety population; hypertension was more frequent with APA + AAP vs AAP mainly in pts ≥ 75 y (31.7% vs 17.6%). Grade 3/4 TEAEs (APA + AAP vs AAP): VD, 60.8%, n = 74 vs 48.5%, n = 68; ≥ 75 y, 71.5%, n = 186 vs 68.5%, n = 165; overall, 63.3%, n = 490 vs 56.2%, n = 489. TEAEs leading to discontinuation: VD, 17.6% vs 5.9%; ≥ 75 y, 26.3% vs 20.6%; overall, 16.9% vs 12.5%. TEAEs leading to death: VD, 6.8% vs 5.9%; ≥ 75 y, 5.4% vs 13.9%; overall, 3.5% vs 7.6%.
Conclusions
In this analysis of two difficult to treat subgroups, addition of APA to AAP favored rPFS and OS. Safety, while generally consistent with the overall population, showed higher hypertension rate in ≥ 75 y and TEAEs leading to discontinuation in VD. Clinical trial information: NCT02257736