Research Funding
Pharmaceutical/Biotech Company
Clovis Oncology, Inc
Background
TRITON3 (NCT02975934) is a randomized, multicenter, open-label, phase 3 study of rucaparib vs physician’s choice (docetaxel [DTX], abiraterone [ABI] or enzalutamide [ENZ]) in patients (pts) with chemotherapy-naïve mCRPC with BRCA1/2 (BRCA) or ATM alterations. It was previously reported that rucaparib significantly improved the primary endpoint of radiographic progression-free survival (rPFS) vs physician’s choice in pts with BRCA or ATM alterations. We report here the interim overall survival (OS) and also report on efficacy of rucaparib compared individually with either DTX or ABI/ENZ.
Methods
Pts had disease progression after 1 prior second-generation androgen pathway inhibitor therapy in any setting and were randomized 2:1 to rucaparib 600 mg BID or physician’s choice of DTX, ABI or ENZ. An ordered step-down multiple comparisons procedure was used to control the overall error rate. OS was a key secondary endpoint with rPFS as the primary endpoint tested first in the BRCA subgroup, then the intent-to-treat (ITT) population. Subgroup analyses based on physician’s choice were exploratory.
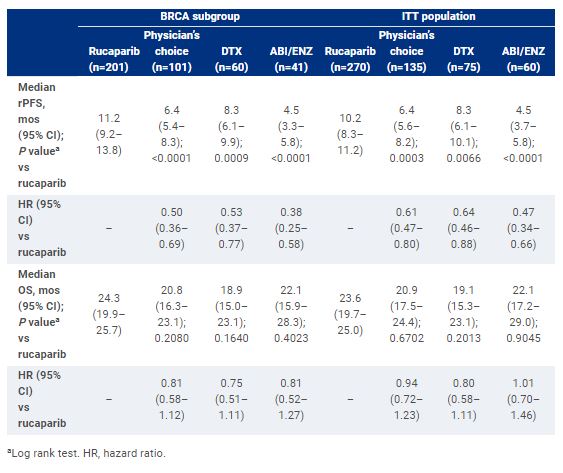
Results
As of August 25, 2022, 302 pts with BRCA and 103 pts with ATM alterations were randomized. OS maturity was 54% in the BRCA subgroup and 59% in the ITT population. rPFS and OS are shown. The most frequent treatment-emergent adverse event (TEAE) in the rucaparib, DTX and ABI/ENZ groups was asthenia/fatigue (61.1%, 67.6% and 57.6%, respectively). The most frequent grade ≥3 TEAE in the rucaparib, DTX and ABI/ENZ groups was anemia (23.7%), neutropenia (14.1%), and hypertension (10.2%), respectively.Conclusions:Rucaparib significantly improved rPFS vs either DTX or ABI/ENZ; safety was consistent with prior reports. Interim OS results suggest a trend towards improvement for rucaparib vs DTX or ABI/ENZ in pts with mCRPC and BRCA alterations. Clinical trial information: NCT02975934.