Background
Both TITAN and ARCHES studies have demonstrated significant clinical benefits of second-generation androgen receptor inhibitors (ARIs) plus ADT versus placebo plus ADT in the treatment of mHSPC. However, first-generation ARIs plus ADT is also widely used in clinic and how superior second-generation ARIs is to first-generation ones remains to be determined. Here, we evaluated the efficacy and safety of SHR3680, a novel oral ARI, versus bicalutamide (Bica) in high-volume mHSPC.
Methods
CHART is a randomized, open-label, phase 3 study (NCT03520478). Patients (pts) with mHSPC were randomized 1:1 to ADT plus either SHR3680 (240 mg/d) or Bica (50 mg/d). All pts had high-volume disease adapted from the CHAARTED study. The primary endpoints were radiographic progression-free survival (rPFS) assessed by independent review committee (IRC) and overall survival (OS). As of May 16, 2021, 209 rPFS events per IRC and 153 deaths occurred and a preplanned interim analysis for rPFS was done.
Results
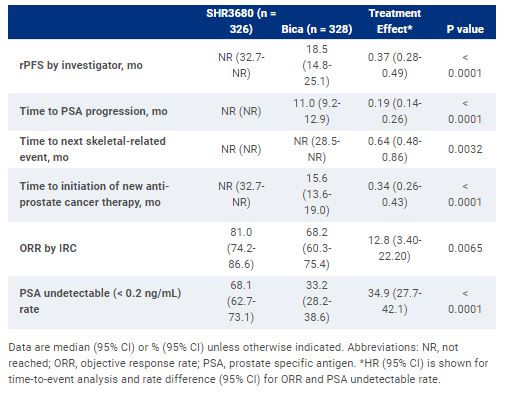
654 pts were randomized to receive SHR3680 (n = 326) or Bica (n = 328). At data cutoff, the median follow-up duration was 22.1 mo in SHR3680 group and 20.4 mo in Bica group. SHR3680 significantly reduced the risk of radiographic progression or death than Bica (HR, 0.44; 95% CI, 0.33-0.58; p < 0.0001; median, not reached vs 25.1 mo). OS data were immature but an improved OS was observed in SHR3680 group compared to Bica group (HR, 0.58; 95% CI, 0.42-0.80; p = 0.0009). All secondary efficacy endpoints favored SHR3680 plus ADT (Table). Frequencies of adverse events of any cause in any grade were similar between groups. Grade ≥3 treatment-related adverse events occurred in 19.2% and 13.9% of pts in SHR3680 and Bica groups, respectively. No seizure occurred in SHR3680 group.
Conclusions
SHR3680 plus ADT significantly improved rPFS versus Bica plus ADT in pts with high-volume mHSPC, with a desirable safety profile. New drug application has been submitted to seek approval based on the data presented here. Clinical trial information: NCT03520478.