Research Funding
This study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Medical writing and editorial assistance were provided by Folabomi Oladosu, PhD, and Jane Beck, MA, from Complete HealthVizion, funded by the study sponsors
Background
In ARCHES (NCT02677896), ENZA + ADT reduced the risk of radiographic progression and improved secondary clinical outcomes in patients with metastatic hormone-sensitive prostate cancer (mHSPC) with variable patterns of disease spread over placebo (PBO) + ADT. This post hoc analysis aimed to evaluate the efficacy of ENZA + ADT in patients with bone oligometastatic mHSPC compared to polymetastatic mHSPC in ARCHES.
Methods
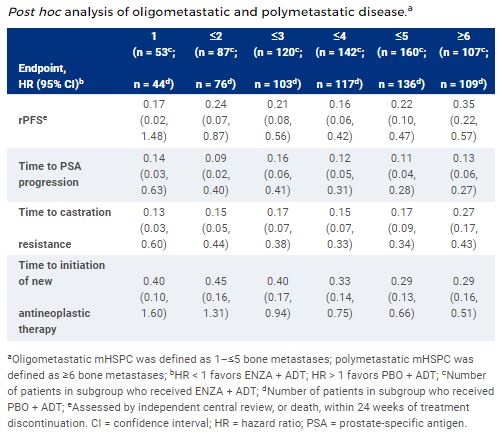
Patients with mHSPC (n = 1150) were randomized 1:1 to ENZA (160 mg/day) + ADT or PBO + ADT, stratified by disease volume and prior docetaxel chemotherapy. This post hoc analysis included patients with bone metastases only, categorized as oligometastatic (1–≤5 metastases) or as polymetastatic (≥6 metastases) based on central review at screening. Efficacy outcomes were compared across treatment arms.
Results
Of the ARCHES population with bone metastases (n = 512), the largest subgroup included patients with ≤5 metastases (ENZA + ADT, n = 160; PBO + ADT, n = 136). Baseline characteristics were generally comparable between treatment arms and across subgroups. When compared to PBO + ADT, ENZA + ADT improved rPFS and secondary endpoints in patients with ≤5 metastases (Table). Similar results were observed across other oligometastatic subgroups (1–≤4) as well as in polymetastatic disease (≥6). The safety profile of ENZA + ADT versus PBO + ADT was similar across subgroups and consistent with previous findings.
Conclusions
This post hoc analysis demonstrates that ENZA + ADT provides clinical benefit across bone oligometastatic as well as polymetastatic mHSPC, supporting the utility of ENZA irrespective of metastatic burden in the ARCHES study. Clinical trial information: NCT02677896