Introduction and objective
In ARCHES (NCT02677896), enzalutamide (ENZA)+androgen deprivation therapy (ADT) improved radiographic progression-free survival and key secondary endpoints vs. placebo (PBO)+ADT for patients (pts) with metastatic hormone-sensitive prostate cancer (mHSPC), also known as metastatic castration-sensitive prostate cancer, irrespective of prior local treatment. Final overall survival (OS) results confirmed a long-term survival benefit with ENZA+ADT in the overall study population (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.53, 0.81; p<0.0001). We report a post hoc analysis of OS in pts with prior local therapy, defined as previous radical prostatectomy (RP) and/or radiation therapy (RT) to the prostate area (definitive, adjuvant, or salvage).
Methods
Pts with mHSPC (n=1150) were randomized 1:1 to ENZA (160 mg/day)+ADT (n=574) or PBO+ADT (n=576), stratified by disease volume and prior docetaxel use. After unblinding, 180 (31.3%) PBO+ADT‒treated pts crossed over to open-label ENZA+ADT. Median OS and HRs were estimated by Kaplan-Meier methods and Cox proportional hazards, respectively. Further analyses by type of prior local therapy (RP, RT, and type of RT) were performed.
Results
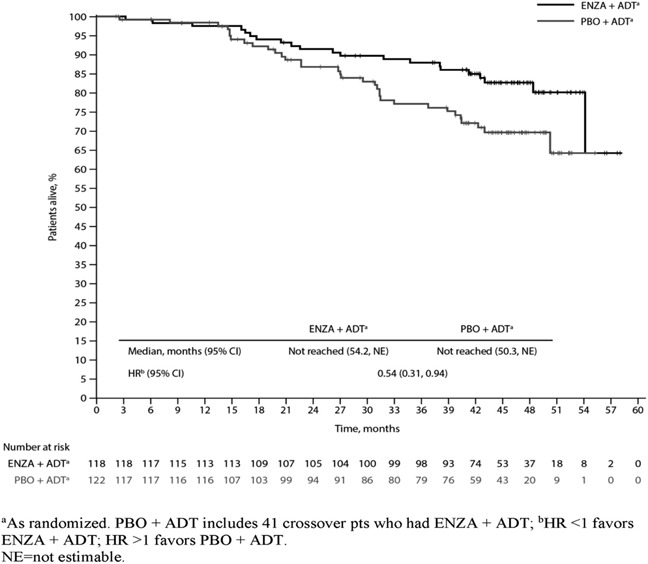
Median treatment duration was 40.2 months (mo) with ENZA+ADT, 13.8 mo with PBO+ADT, and 23.9 mo with ENZA+ADT in crossover pts. Median follow-up time was 44.6 mo. Including crossover, 401 (69.9%) PBO+ADT pts had a subsequent life-prolonging therapy. Prior local therapy was reported in 118 ENZA+ADT (45 RP, 46 RT, 27 both) and 122 PBO+ADT (50 RP, 33 RT, 39 both) pts; of these, 21 (17.8%) and 33 (27.0%) died, respectively. ENZA+ADT reduced the risk of death by 46% vs. PBO+ADT in pts with prior local therapy (HR 0.54; 95% CI 0.31, 0.94) [Figure], consistent with the overall study population. Median OS was not reached for either arm.
Conclusions
Our post hoc analysis demonstrates the long-term survival benefit of ENZA+ADT vs. PBO+ADT in pts with mHSPC who received prior local therapy, despite substantial treatment crossover and subsequent therapy use in PBO+ADT pts.
Source of Funding
This trial was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Medical writing and editing assistance were provided by Jake Stoddart, MRes, and Lauren Smith, BA (Hons), from Complete HealthVizion, funded by the trial sponsors