Background
[177Lu]Lu-PSMA-617 (177Lu-PSMA-617) delivers β-particle radiation to prostate-specific membrane antigen (PSMA) expressing cells and the surrounding microenvironment. In the phase III VISION study (NCT03511664), 177Lu-PSMA-617 + protocol-permitted standard of care (SOC) prolonged radiographic progression-free survival (rPFS; HR, 0.40; 99.2% CI: 0.29, 0.57), overall survival (OS; 0.62; 95% CI: 0.52, 0.74) and time to first symptomatic skeletal event (SSE; 0.50; 95% CI: 0.40, 0.62) versus SOC (all p < 0.001).
Methods
VISION was an international, open-label study of 177Lu-PSMA-617 in adults with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) previously treated with ≥ 1 androgen receptor pathway inhibitor and 1–2 taxane regimens. Patients were randomized 2:1 to 177Lu-PSMA-617 (7.4 GBq every 6 weeks, ≤ 6 cycles) plus SOC or to SOC alone. rPFS and OS were alternate primary endpoints; time to SSE was a key secondary endpoint. Other secondary endpoints included safety and patient-reported HRQoL (Functional Assessment of Cancer Therapy – Prostate [FACT-P]) and pain (Brief Pain Inventory – Short Form [BPI-SF]). Pre-specified analyses included time to the first occurrence of HRQoL/pain worsening, disease progression or death. Ad hoc analyses included time to worsening only (non-inferential).
Results
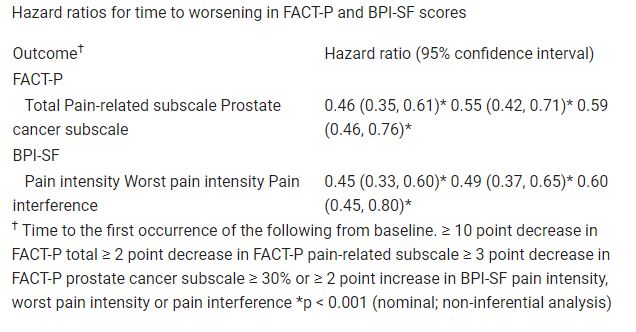
HRQoL was assessed in the pre-specified rPFS analysis set comprising 581 of the 831 randomized patients (177Lu-PSMA-617 arm, n = 385; control arm, n = 196). HRQoL and pain time-to-worsening analyses favoured the 177Lu-PSMA-617 arm (Table), despite a higher incidence of grade ≥ 3 adverse events versus SOC alone. No new or unexpected safety concerns were noted, including changes in creatinine clearance. Table: 576MO
Conclusions
177Lu-PSMA-617 plus SOC was generally well tolerated and delayed time to HRQoL and pain worsening versus SOC alone in patients with advanced mCRPC.
Clinical trial identification
PSMA-617-01. 08 July 2019. EudraCT 2018-000459-41.