Research Funding
Pharmaceutical/Biotech Company
This study was supported by AstraZeneca and Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who are codeveloping olaparib
Background
PROpel (NCT03732820) met its primary endpoint showing significant investigator-assessed radiographic progression-free survival (rPFS) benefit for patients with mCRPC treated with abi + ola vs abi + pbo in the 1L setting (hazard ratio [HR] 0.66, 95% confidence interval [CI] 0.54–0.81, P< 0.001, data cut-off: 7/30/2021). Sensitivity analysis by blinded independent central review was consistent. A trend toward OS benefit with abi + ola was observed at the time of the primary rPFS analysis (28.6% maturity, HR 0.86, 95% CI 0.66–1.12) and a subsequent interim analysis (40.1% maturity, HR 0.83, 95% CI 0.66–1.03). We report OS and safety from the pre-planned final analysis (data cut-off: 10/12/2022).
Methods
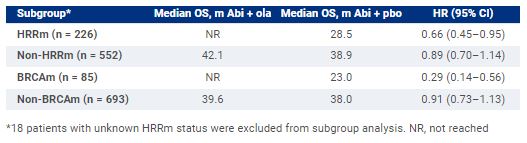
PROpel is a randomized, double-blind phase 3 trial of 1L therapy for patients with mCRPC eligible for abiraterone. Patients were prospectively assessed for homologous recombination repair mutation (HRRm) status using tumor tissue (FoundationOneCDx) and/or circulating tumor DNA (ctDNA; FoundationOneLiquid CDx) tests after randomization 1:1 to ola (300 mg twice daily [bid]) or pbo, and abi (1000 mg once daily) plus prednisone/prednisolone (5 mg bid). Treatment continued until radiographic disease progression, unacceptable toxicity or withdrawal of consent. OS was a key secondary endpoint (2-sided boundary for significance 0.0377). Aggregate results from tumor tissue and ctDNA tests were used to assign patients to HRRm/BRCAm subgroups.Results:Patient (n = 796) characteristics (including prior docetaxel, site of metastasis, symptom score and HRRm status) were generally balanced. There was a consistent trend toward OS benefit in the intention-to-treat (ITT) population with abi + ola vs abi + pbo (maturity 47.9%, HR 0.81, 95% CI 0.67–1.00, P= 0.0544), with median OS 42.1 months (m) vs 34.7 m, respectively. OS medians and HRs for HRRm, non-HRRm, BRCAm and non-BRCAm subgroups all favored abi + ola vs abi + pbo. In the abi + ola arm the most common Grade ≥3 adverse event was anemia (16.1%).
Conclusions
At the prespecified final analysis in PROpel, abi + ola prolonged OS by > 7 m vs standard-of-care abiraterone (abi + pbo) in the ITT population. The median OS of > 42 m is the longest median reported to date in a phase 3 trial in 1L mCRPC. Consistent with rPFS results, a trend toward OS benefit was observed in HRRm, non-HRRm, BRCAm and non-BRCAm subgroups with greatest benefit in the BRCAm subgroup. No new long-term safety issues were identified. These results support the use of abi + ola in 1L mCRPC. Clinical trial information: NCT03732820.