Background
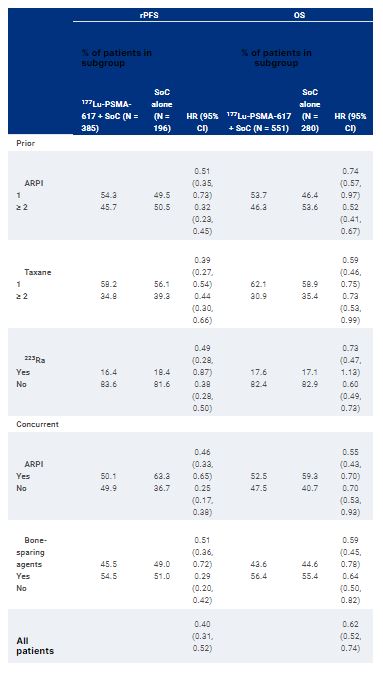
In the phase 3 VISION trial, targeted radioligand therapy with lutetium (177Lu) vipivotide tetraxetan ([177Lu]Lu-PSMA-617; 177Lu-PSMA-617) significantly prolonged radiographic progression-free survival (rPFS) and overall survival (OS) when added to standard of care (SoC) in patients with advanced prostate-specific membrane antigen (PSMA) PET-positive metastatic castration-resistant prostate cancer. Benefits were consistent across most pre-specified subgroups. In this post hoc exploratory analysis, we examined rPFS and OS in the context of prior and concomitant cancer-directed treatments.
Methods
In VISION, adult patients previously treated with at least 1 androgen receptor pathway inhibitor (ARPI) and 1–2 taxane regimens were randomized 2:1 to 177Lu-PSMA-617 (7.4 GBq Q6W, up to 6 cycles) + SoC or SoC alone. Protocol-permitted SoC excluded cytotoxic chemotherapy, systemic radioisotopes, immunotherapy, or other investigational drugs. Exploratory subgroup analyses of rPFS and OS were performed by: number of prior ARPIs; taxane regimens; non-taxane regimens and immunotherapies; prior treatment with bone-sparing agents; 223Ra and PARP inhibitors; and concomitant treatment with ARPIs, radiation therapy, and bone-sparing agents.
Results
Prior and concomitant treatments were generally well balanced between study groups (Table). rPFS and OS benefits with 177Lu-PSMA-617 were consistent across all prior treatment subgroups. Notably, there were benefits in patients who had not received a second prior taxane. There were also consistent benefits regardless of concomitant systemic and radiation therapy as part of SoC.
Conclusions
The clinical efficacy of 177Lu-PSMA-617 was observed regardless of prior treatment or SoC chosen, suggesting that disease biology rather than prior and concomitant treatment context drives outcomes. Small differences in outcomes between subgroups may warrant further study to understand better the predictors of improved clinical benefit. Clinical trial information: NCT03511664.